Ice melting (pure water)
Introduction
Ice melting is probably one of the most common phase transition in fluid inclusions as many natural fluids are composed of an aqueous saline solution.
Video
The following video presents the cooling of a synthetic fluid inclusion composed of pure water (provided by Ronald Bakker, University of Leoben, Austria).
The expected ice melting temperature is therefore 0.0°C.
Video description
At the beginning of the experiment (room temperature), the fluid inclusion is two-phase, including a vapour bubble (a very low density water steam, quasi-vacuum) and a liquid phase.
During cooling no apparent change occurs (except an indistinguishable bubble size increase). The 0°C limit is crossed, but without any change.
At about -36°C a slight change occurs: The vapour bubble diameter slightly decreases (volume decrease = contraction). This change is linked to the transformation of liquid water into ice.
This phase transition is associated to a volume increase (the ice density is more than the liquid water density).
During heating no change occurs up to a few degrees below zero. At about -2 to -1°C the ice aspect changes inducing a significant change of the vapour meniscus (irregularities, pits,...).
This is probably related to recrystallization of the ice crystals.
At 0.0°C ice melts suddenly. This is exactly the expected temperature for ice melting in pure water.
Following heating up to room temperature will not induce any other change.
Interpretation
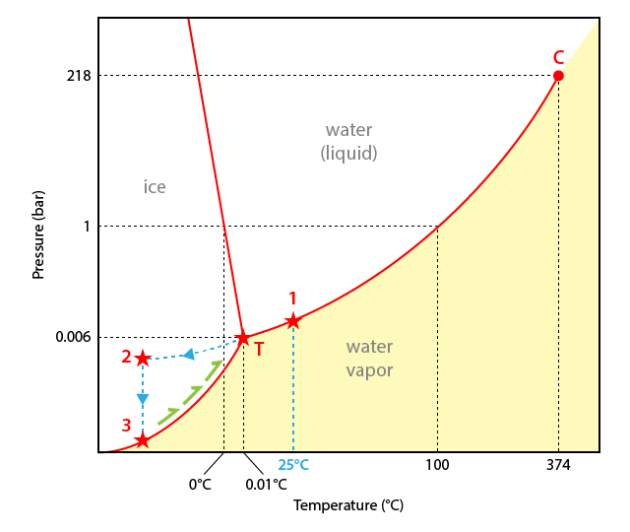
The experiment will be interpreted in a temperature-pressure diagram for pure water.
The presence of a liquid and a vapour at the experiment start (25°C) induces that the representative point of the inclusion content is located on the liquid+vapour curve (point#1).
During cooling the liquid+vapour equilibrium is maintained and the inclusion follows the liquid+vapour curve down to the triple point (point T).
At the conditions of point T, ice should theoretically appear. However for metastability reasons ice does not nucleate and the inclusion content goes on following a metastable extension of the liquid+vapour curve down to about -36°C. Keeping liquid water in conditions where it does not theoretically exist is called undercooling (or supercooling).
At point#2 (about -36°C) ice nucleates. The inclusion content jumps down to the ice+vapour curve (sublimation curve) (point#3).
During heating the inclusion follows the ice+vapour curve up to 0.0°C (point T). Ice therefore melts suddenly as point T is an invariant point.
Going on heating will not induce any other change.
More ...
Ice melting temperature of 0°C in natural fluid inclusions indicates undoubtedly the trapping of pure water. Pure water is documented in low depth environments and the origin is generally meteoric water (rain) or a sub-surface aquifer. Pure water can also result from the condensation of a vapour phase produced by boiling of a saline fluid around a magmatic chamber.